Introduction:
Donor lymphocyte infusion (DLI) is a form of adoptive immunotherapy administered to enhance the graft-versus-leukemia (GVL) effect after an allogeneic hematopoietic cell transplantation (HCT). DLI is frequently chosen for its ease of administration and low risk of graft ablation as compared with other chemotherapeutic options in patients experiencing mixed chimerism (MC) or disease relapse. But in patients with acute leukemia experiencing relapse, clinical responses are seen in fewer than 20-30%. Success is also limited by the occurrence of acute and chronic graft-versus-host disease (GVHD). We had previously described our risk-adapted strategy of administering DLIs based on chimerism and donor source (Rujkijyanont P, et al. Blood Cancer J 2013). To determine the efficacy and toxicity of DLIs and to identify potential factors influencing the outcomes, we conducted a retrospective single center study including patients who received DLIs after HCT.
Methods:
We collected data on all HCTs and conventional DLIs that had been administered to patients from 1993 to 2022 for any indication at our center. Each HCT was considered as an independent event. Numeric variables were represented by median and range and compared using the Wilcoxon-Mann-Whitney test. Categorical variables were compared using the Fisher exact test. Competing-risk regression models using the Fine-Gray method were developed to evaluate risk factors for acute GVHD and chronic GVHD. Death and relapse were considered as competing risk events for these GVHD outcomes. Follow-up data were censored at subsequent HCT, 2 years after the first DLI, 5 years after transplant, or the last follow-up. Logistic regression analysis was performed to assess factors associated with the response to DLI within 180 days after the first DLI.
Results:
During the study period, 236 patients received 252 HCTs followed by DLIs (median age at HCT 9.2 [range 0.2-25.1] years). An additional 743 patients received 806 HCTs with no subsequent DLI (median age at HCT 10.3 [range 0.1-27.2] years). Dosing most frequently used was 1 × 10 5, 1 × 10 6 and 1 × 10 7 CD3+ cells/kg, for haploidentical donor (HAPLO), matched unrelated donor (MUD) and matched sibling donor (MSD) recipients, respectively. Initial doses were occasionally lower based on risk of GVHD and were escalated as tolerated and based on response.
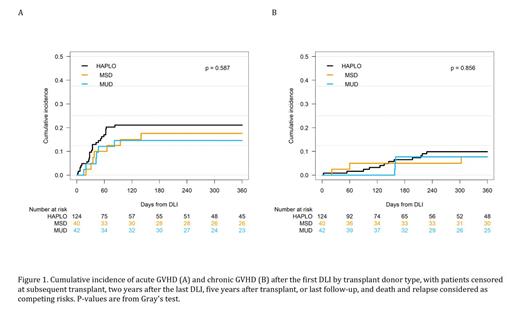
After receiving DLI, 19.4% developed acute GVHD, and 9.2% developed chronic GVHD with no significant differences observed between MSD, MUD and HAPLO recipients. After adjusting for donor source, longer duration between HCT and DLI was associated with a significantly decreased risk of both acute (HR 0.97 P<0.001) and chronic GVHD (HR 0.98 P=0.04). A higher CD3+ cell dose was also significantly associated with an increased risk of acute GVHD (HR 1.03 P<0.001) but not chronic GVHD (HR 0.96 P=0.12).
The overall response rate after DLI for treating MC or graft failure was 45.2% for MSD, 32.6% for MUD and 41.4% for HAPLO recipients. Logistic regression analysis revealed leukemia patients had a higher response rate after receiving a DLI for mixed chimerism from MSD (OR=19.5 P=0.002) and HAPLO (OR=9.03 P=0.042), but not from MUD HCT (OR=0.65 P= 0.57), compared to patients with non-malignant disorders. We also evaluated the response to DLI for frank disease relapse in 15 patients. The response rate after DLI for disease relapse was 50% (1/2) for MSD, 44.4% (4/9) for HAPLO, but 0% (0/4) for MUD recipients.
Conclusions:
In our analysis, the incidence of GVHD was not higher in pediatric and young adult HCT recipients who received DLI as compared to those who did not. Overall, the risk of developing acute and chronic GVHD after DLI was low and comparable between different donor sources. The risk of GVHD decreased further away from HCT and increased with higher DLI dose. The response rate for treating MC and disease relapse remains less than 50%. Thus, DLIs may be safely administered to pediatric and young adult patients after HCT. The therapeutic effect may be modulated by adjusting the dose, timing and frequency of DLI administration.
Disclosures
Gottschalk:Sanofi: Consultancy; TESSA Therapeutics: Consultancy; Immatics: Other: Member of the Data Safety Monitoring Board; BeBiopharma: Membership on an entity's Board of Directors or advisory committees. Sharma:Vertex Pharmaceuticals: Consultancy, Other: Clinical Trial Site PI; Sangamo Therapeutics: Consultancy; Editas Medicine: Consultancy; CRISPR Therapeutics: Other: Clinical Trial Site PI, Research Funding; RCI BMT/NMDP: Honoraria, Other: Clinical Trial Medical Monitor; Medexus Inc: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal